Applications
This tool has already been used to investigate the effects of various membrane parameters on interfacial properties, including acyl chain mono- and poly-unsaturation, membrane curvature, and the presence of conical lipids or of molecules against lipointoxication. Of note, a pivotal role of interfacial voids has been suggested by a number of recent investigations in disparate topics, including membrane remodeling processes such as bending, fusion and fission, as well as the binding of peripheral proteins, disaccharides and nanoparticles. Considering the ever-growing interest in cellular membranes and in their interactions with proteins and nanoparticles, PackMem should provide a useful tool to investigate membrane interfacial properties at the structural level and for a large community of computational scientists.
Bending and fission of membranes
Phospholipids (PLs) with polyunsaturated acyl chains are extremely abundant in a few specialized cellular organelles such as synaptic vesicles and photoreceptor discs. It has been found that polyunsaturated PLs increase the ability of dynamin and endophilin to deform and vesiculate synthetic membranes. When cells incorporated polyunsaturated fatty acids into PLs, the plasma membrane becomes more amenable to deformation by a pulling force and the rate of endocytosis is accelerated, in particular, under conditions in which cholesterol is limiting. Molecular dynamics simulations and biochemical measurements indicate that polyunsaturated PLs adapt their conformation to membrane curvature. Thus, by reducing the energetic cost of membrane bending and fission, polyunsaturated PLs may help to support rapid endocytosis.
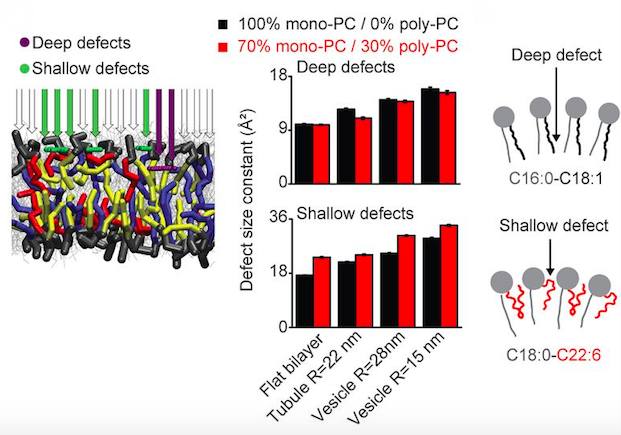
(Left) Characteristic area constant of deep and shallow lipid-packing defects in bilayers of the indicated shape and containing 0 or 30 mol % polyunsaturated PC. (Right) Models showing that polyunsaturated PLs adapt their conformation to membrane curvature, thereby favoring the formation of shallow lipid-packing defects at the expense of deep ones. (from Pinot et al. 2014 DOI: 10.1126/science.1255288)
Curvature
Two parameters of biological membranes, curvature and lipid composition, direct the recruitment of many peripheral proteins to cellular organelles. The correlation between the membrane partitioning of model amphipathic helices and the distribution of lipid-packing defects in membranes of different shape and composition explains how macroscopic membrane properties modulate protein recruitment by changing the molecular topography of the membrane interfacial region.
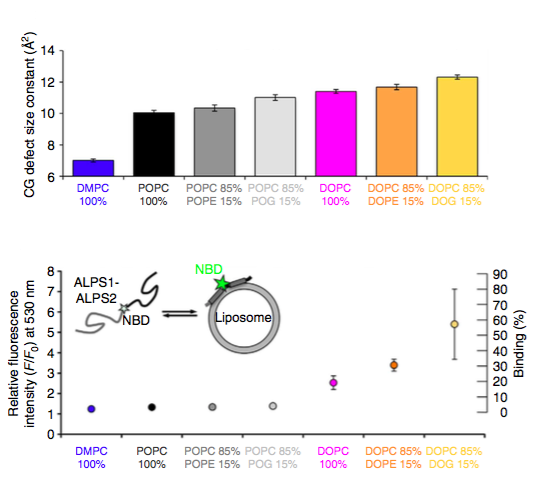
(Top) Defect size constants as determined from linear fits in CG simulations of bilayers of different compositions. (Bottom) Membrane partitioning of NBD-labelled ALPS1-ALPS2 in the presence of large liposomes of the same lipid compositions as those studied by MD. (From Vanni, Hirose et al. 2014 DOI: 10.1038/ncomms5916)
Branched Lipids
Alpha-synuclein (AS) is a synaptic protein that is involved in Parkinson's disease due to its tendency to form protein aggregates. Since AS aggregation can be dependent on the interactions between the protein and the cell plasma membrane, elucidating the membrane binding properties of AS is of crucial importance to establish the molecular basis of AS aggregation into toxic fibrils. All-atom molecular dynamics simulations was used to investigate the influence of methyl-branched lipids on interfacial membrane properties. Is has been found that methyl-branched lipids promote the membrane adsorption of AS by creating shallow lipid-packing defects to a larger extent than polyunsaturated and monounsaturated lipids. These findings suggest that methyl-branched lipids may constitute a remarkably adhesive substrate for peripheral proteins that adsorb on membranes via hydrophobic insertions.
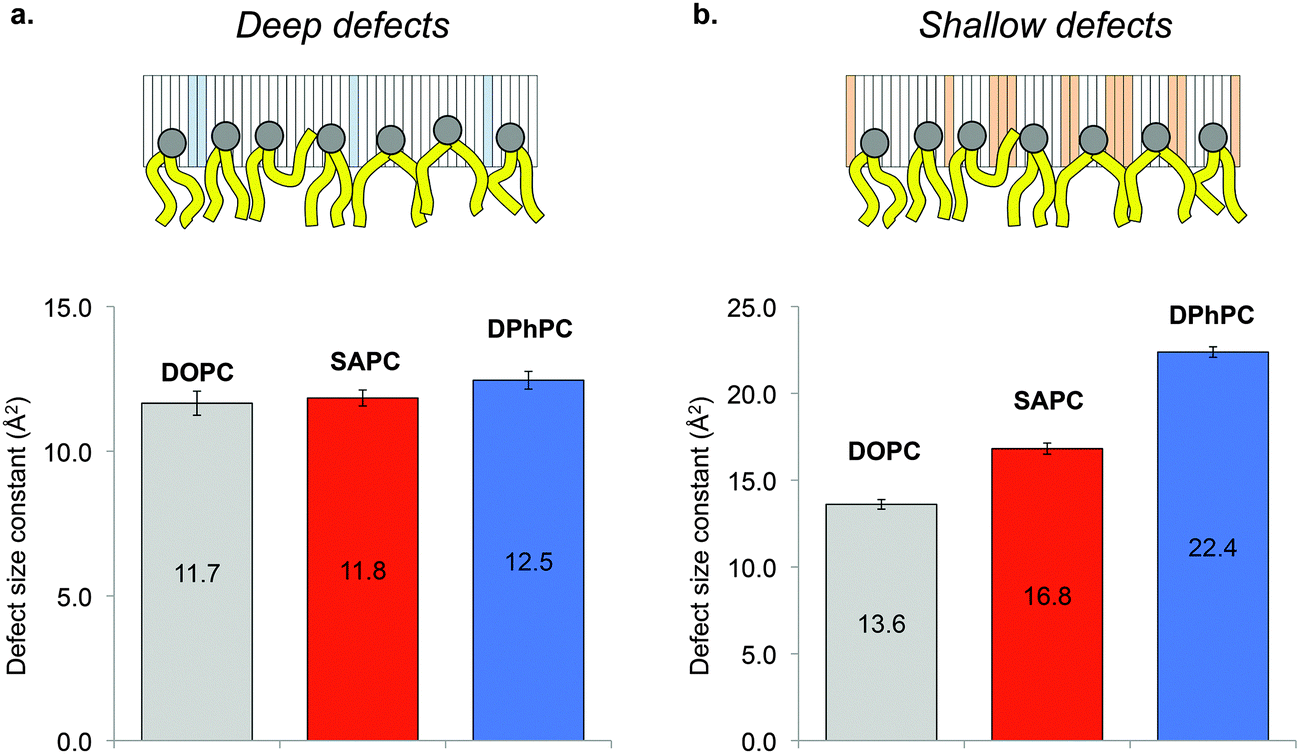
Lipid-packing defects in all-atom molecular dynamics simulations. Deep (a) and shallow (b) lipid packing defects in all-atom molecular dynamics simulations of DOPC, SAPC and DPhPC lipid bilayers. The defect size constants shown are the fit to the single exponential distribution obtained by plotting the defect occurrence as a function of their size. The prefactors of the exponential fit are identical for the three lipid composition. (from Garten et al. 2015 DOI: 10.1039/C5CP00244C)
Lipointoxication
Saturated fatty acids (SFA), which are abundant in the so-called western diet, have been shown to efficiently incorporate within membrane phospholipids and therefore impact on organelle integrity and function in many cell types. The compounds identified here were effective in relieving lipointoxication in mammalian β-cells, one of the main targets of SFA toxicity in humans. In vitro reconstitutions and molecular dynamics simulations on bilayers revealed that these molecules, albeit according to different mechanisms, can generate voids at the membrane surface. The resulting surface defects correlate with the recruitment of loose lipid packing or void-sensing proteins required for vesicular traffic, a central cellular process that is precluded under SFA accumulation.
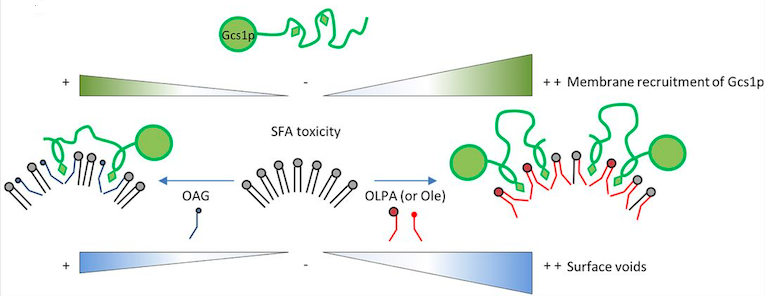
Schematic representation summarizing OLPA or Ole, and OAG actions on SFA-overloaded membranes. Whereas OLPA and Ole efficiently metabolize their UFA moiety within membrane phospholipids to generate diunsaturated species, OAG might preferentially intercalate within saturated membranes at first glance to act as a nanospacer. Both mechanisms restore surface voids compatible with amphipathic-lipid-packing sensor (ALPS)-motif-containing proteins – such as Gcs1p in yeast – recruitment, membrane vesiculation and protein trafficking along the secretory pathway. (from Ferru-Clement et al. 2016 DOI: 10.1242/jcs.183590)
